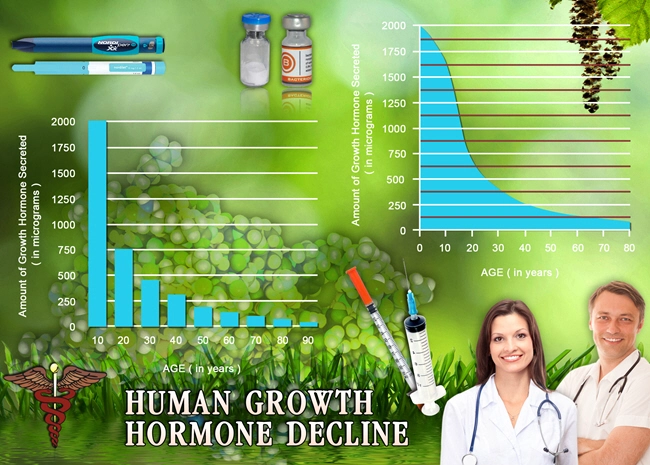
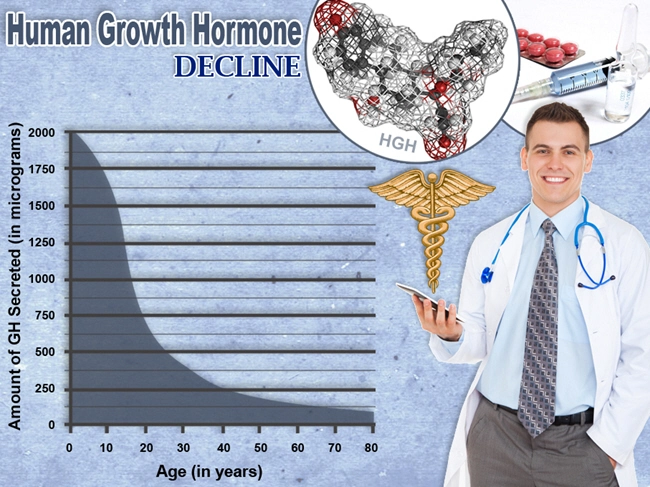
Description:
Saizen is a Human Growth Hormone somatropin injection derived from recombinant DNA technology. Saizen is comprised of 191 residue amino acids and has a molecular weight of 22,125 daltons. The sequence of Saizen's amino acids is completely identical to the sequence of Human Growth Hormone derived naturally from the pituitary.
Saizen is created from a specific lab strain of the E. coli bacteria as a precursor chemical that consists of the recombinant Human Growth Hormone molecule that is preceded by a signal for secretion that originates with a protein from the E. coli bacteria. This precursor chemical is then directed to the plasma membranes of  the cells. After the E. coli secretion signal is eliminated, the native protein is deposited into the periplasm and the protein begins to be folded correctly as it is synthesized.
the cells. After the E. coli secretion signal is eliminated, the native protein is deposited into the periplasm and the protein begins to be folded correctly as it is synthesized.
Saizen is a pristine purified preparation. The biological potency of Saizen is configured through a cell proliferation bio assay. Upon expiration, Saizen will not contain more than 15% deamidated GH upon its expiration. Deamidate growth Hormone has been studied extensively, and it has been proven to be safe and completely active.
Saizen is a sterilized liquid that is intended to be administered with a subcutaneous injection. Saizen is almost isotonic at a 5mg GH per mL concentration. It has a pH value of around 6.0.
Every 2 mL container of Saizen holds10mg (about 30 IU) of somatropin formulated in 17.4 mg NaCl, 4 mgpolysorbate 20, 5 mg phenol, and 10mM sodium citrate.
Clinical Pharmacology
General Information
In vivo and in vitro clinical and preclinical testing has provided ample evidence that Saizen is equivalent therapeutically to Human Growth Hormone naturally derived from the pituitary. Children who do not secrete adequate Growth Hormone levels endogenously, patients who suffer from renal insufficiency that is chronic in nature, and patients who are realigned with Turner syndrome all report benefits when taking Nutropin or Saizen [injectable somatropin derived from recombinant DNA].
Patients who took Nutropin or Saizen were found to experience an increase in their rate of growth and also an increase in their IGF-1 (insulin-like growth factor-I) levels in a manner that is also seen when normal Human Growth Hormone levels are naturally produced by the pituitary gland.
Benefits that have been shown through studies regarding Saizen, somatrem, somatropin, and/or Human Growth Hormone derived naturally from the pituitary include:
A. Tissue Growth --
1) Skeletal Growth: Growth Hormone stimulates skeletal growth in
young patients that experience growth failure as a result of inadequate secretion of natural Growth Hormone, or as a secondary effect of chronic kidney insufficiency, or also with patients that suffer from Turner syndrome. Growth of the long bones of the skeleton is a result of the expansion of the epiphyseal plates that are located on the ends of the growing bone. The metabolism and growth of the cells of the epiphyseal plates are stimulated directly by Growth Hormone and insulin-Like Growth Factor-1, an important stimulator of growth hormone.
Levels of IGF-1 in the blood serum are low in adolescents and children who are deficient in GH, but their levels increase when treated with Growth Hormone. In these young patients, the new bone begins to develop through the epiphyses after Growth Hormone and IGF-1 have been introduced to the body. As one reaches the end of puberty, the growth plates fuse and adult height is locked in.
2) Cell Growth: Human Growth Hormone leads to an increase in both the size and number of skeletal muscle cells.
3) Organ Growth: Growth Hormone has a direct effect on the size of the internal organs, including the kidneys. It also increases the mass of red cells. Growth Hormone Treatment of genetic dwarf or hypophysectomized rats leads to organ development that is in proportion to the growth of the rest of the body. In healthy rats that were induced with uremia through nephrectomy, Growth Hormone promoted body and skeletal growth.
B. Protein Metabolism Proper growth is stimulated in part by protein synthesis which is encouraged by Growth Hormone. This is shown through retention of nitrogen that is demonstrated by a lower level of nitrogen excreted through the urine, as well as clinical testing for nitrogen in the blood urea which occurs during Growth Hormone Therapy.
C. Carbohydrate Metabolism Growth Hormone is a significant controlling mechanism in charge of carbohydrates. In patients that do not adequately secrete Growth Hormone, hypoglycemia is often experienced during periods of fast. This issue is largely resolved with Human Growth Hormone Therapy. It is a possibility that Growth Hormone therapy can lead to a decrease in the body's sensitivity to insulin. Patients who are not treated for chronic kidney insufficiency and/or Turner syndrome have a higher risk to develop intolerance to glucose.
Human Growth Hormone treatment provided to children or adults has resulted in an increase in postprandial insulin levels and serum fasting. These two results happen most commonly with those that are overweight or obese. Also, postprandial glucose, mean fasting, and hemoglobin A 1c levels stay within a normal range.
D. Lipid Metabolism In patients who are Growth Hormone Deficient, Growth Hormone Therapy led to the mobilization of lipids, a reduction in stores of body fat, increased fatty acids entering the plasma, and lower levels of cholesterol in the plasma.
E. Mineral Metabolism Use of Growth Hormone leads to the retention of potassium in the body. This retention apparently is due to the enhanced cell growth that begins to occur.
Inorganic phosphorus levels in the blood serum can sometimes increase modestly among patients who undergo Growth Hormone Therapy because they do not  produce enough Growth Hormone on their own, have achronic kidney deficiency, or suffer from Turner syndrome because Growth Hormone enhances the metabolic processes associated with the growth of bone in addition to improving tubular reuptake of phosphate by the kidneys.
produce enough Growth Hormone on their own, have achronic kidney deficiency, or suffer from Turner syndrome because Growth Hormone enhances the metabolic processes associated with the growth of bone in addition to improving tubular reuptake of phosphate by the kidneys.
Serum calcium levels do not change significantly among these patients. Retention of sodium also occurs. Adults that developed a Growth Hormone Deficiency as children display low levels of bone mineral density (Acronym: BMD). (See also Precautions: Laboratory Tests.)
F. Connective Tissue Metabolism Growth Hormone chondroitin sulfate synthesis and collagen synthesis in addition to encouraging the urinary expulsion of hydroxyproline.
Pharmacokinetics
Absorption after Subcutaneous Injection The total bioavailability of recombinant Human Growth Hormone after being injected subcutaneously into a healthy adult male is determined to be 81plus or minus 20%. The terminal means after subcutaneous injection is much longer than the terminal mean after intravenous injection (2.1 plus or minus 0.43 hr vs. 19.5 plus or minus 3.1 min). This indicates that subcutaneous injection of Growth Hormone causes the compound to be released slowly and at a more modest rate.
Distribution Recombinant Human Growth Hormone Animal Research has shown that Growth Hormone is attracted to organs that are highly perfused, in particular the kidneys and the liver. The distribution volume at steady state for Recombinant Human Growth Hormone in a healthy adult male is around50 mL/kg of body weight, essentially the serum volume.
Metabolism Both the kidney and liver have been proven to be essential organs for the proper metabolism of Growth Hormone. Animal research provides evidence that the kidneys are the primary organs of clearance. Growth Hormone filtration occurs at the glomerulus and Growth hormone is reabsorbed by the proximal tubules. After that, it is split inside the renal cells into its component amino acids, after which the acids return to normal circulation.
Elimination--The terminal mean t after intravenous injection of recombinant Human Growth Hormone adult males in good health is approximated to be19.5 plus or minus 3.1 minutes. Elimination of recombinant Human Growth Hormone after intravenous injection among healthy children and adults is shown to range from 116-174 mL/hr/kg.
Formulation bio-equivalence Saizen [somatropin injection derived from recombinant DNA] has been shown to be bioequivalent of Nutropin [somatropin injection derived from recombinant DNA]. This is based upon the statistical evaluation of Area Under the Curve (AUC) and man C.
SpecialPopulations
Pediatric Current research data suggests that there is no difference in bio-identical Human Growth Hormone clearance between children and adults.
Gender There is no data available for exogenously injected recombinant Human Growth Hormone. The data that has been collected regarding methionyl rhGH naturally produced and extracted pituitary Growth Hormone, and endogenous Growth Hormone produces no evidence that suggests that men and women eliminate Growth Hormone at different rates.
Geriatrics The limited amount of published research shows that Growth Hormone plasma clearance and normal steady-state Growth Hormone plasma concentration do not appear to be different among young and elderly populations.
Race The half-life values for endogenous Growth Hormone in healthy black males are not shown to be any different than the half-life values of Growth Hormone for healthy white males. There is no available data regarding other races.
Growth Hormone Deficiency(GHD)The reported values regarding the clearance of recombinant Human Growth Hormone in children and adults suffering from GHD range from 138-245 mL/hr/kg. These values are similar to those that occur in healthy children and adults. The terminal means values following subcutaneous and intravenous administration in pediatric and adult Growth Hormone Deficiency patients have also proven to be similar to the values observed in the population of healthy adult males.
Kidney Insufficiency Adults and children who suffer from end-stage renal disease (ESRD) and chronic renal failure (CRF) clear rhGH at a lower rate than healthy individuals. Endogenous Growth Hormone production can sometimes increase in particular individuals suffering from ESRD. Even so, no accumulation of recombinant Human Growth Hormone has been shown in children with ESRD of CRF that are given normal Growth Hormone Replacement Therapy.
Turner Syndrome No pharmacokinetic data is currently available regarding Turner syndrome and Growth Hormone Therapy. It has been reported, however, that absorption, half-life, and clearance rates for internally produced Growth Hormone for this population show little difference when compared to the rates of healthy patients and patients only suffering from Growth Hormone Deficiency.
Hepatic Insufficiency A reduction in recombinant Growth Hormone clearance has been reported in patients that are suffering from extreme liver dysfunction. The clinical significance of this data is still unknown.
Research on the effectiveness of Saizen
Effect of the Bio-synthetic hormone Nutropin [recombinant DNA derived somatropin for injection]upon Growth Failure as a result of CRI
Two randomized, controlled,multi-center clinical trials were conducted in an effort to determine whether Nutropin treatment before renal transplantation in patients suffering from chronic renal insufficiency could possibly lead to an improvement in growth rate and a decrease in height deficit. One of the studies was a placebo-controlled, double-blind trial and the other trial was randomized and open-label. In both of these controlled studies, participants took a Nutropin dose of 0.05 mg/kg/day (0.35 mg/kg/wk). These doses were given daily by means of subcutaneous injection.
Taking data from both studies of patients who underwent treatment for two full years resulted in 62 participants who received Nutropin treatment and 28participants who were either untreated or treated by placebo. The year one mean growth rate was 10.8 cm/yr for patients treated with Nutropin, as compared with a mean growth rate of 6.5 cm/yr for the untreated/placebo controls (P
Growth after Transplantation
There is a famous study by NAPRTCS that has produced data regarding growth after transplant in pediatric patients who didn't receive Growth Hormone. The average SD score for change in height during the first two years after the transplant was received was 0.18 (300 total patients, Journal of Pediatrics 1993;122:397-402).
Controlled studies of Growth Hormone treatment administered to combat short stature that accompanies CRI were not undergone to compare the growth rates of untreated and treated patients after they underwent a renal transplant. Even so, the growth data is available from a small subset of the patients that were followed for a minimum of 11 months.
Among the 7 control patients, 4 had a higher SD height score and three showed either no change of significance or a decrease in their SD height score. Among the 13 patients that received Nutropin [somatropin for injection derived from recombinant DNA] before their transplant displayed either no change of significance or an increased SD height score post-transplantation.
This indicates that the gains that were achieved with Growth Hormone Therapy before the renal transplant were maintained post-transplantation. The deficit between the heights of the treated and untreated group narrowed as time passed after renal transplant.
Turner Syndrome
One multi-center, concurrently controlled, open-label, long-term randomized study, two multi-center, historically controlled, open-label, long-term studies, and one randomized, dose-response, a long-term study was conducted to test the efficacy of Growth Hormone in treating girls who have short stature as a result of Turner syndrome.
In the randomized GDCT study which compared patients treated with Growth Hormone to a concurrent control group that did not receive Growth Hormone, the  Growth Hormone-treated patients received a dosage of 0.3 mg/kg/wk 6 times each week. The mean age of the participants was 11.7 years and the duration of treatment was a mean of 4.7 years.
Growth Hormone-treated patients received a dosage of 0.3 mg/kg/wk 6 times each week. The mean age of the participants was 11.7 years and the duration of treatment was a mean of 4.7 years.
The group that received therapy reached a mean height of 146.0 (n=27) as they reached near-final height. The control group reached a near-final height of only 142.1 cm (n=19). Through covariant analysis, the total effect of Growth Hormone therapy was an increase in the mean height of 5.4 cm (p value=0.001).
In two of these studies, (85-044 and85-023), the result of long-term Growth Hormone treatment (0.375mg/kg/wk either daily or three times weekly) on final height upon adulthood was figured through a comparison of the final adult height of patients treated with Growth Hormone matched by age with historical controls of Turner syndrome patients who received no therapy to promote growth.
In Study 85-023, estrogen replacement therapy was not given until the patients reached the age of 14. Growth Hormone therapy led to an adult mean height gain of 7.4 cm(duration mean of Growth Hormone therapy=7.6 years) when compared to matched historical controls through covariant analysis.
In Study 85-044, patients that received Growth Hormone therapy early were randomly assigned to receive therapy to replace estrogen (through the use of conjugated estrogen, initial dose 0.3 mg, escalated overtime to 0.625 daily)at either age twelve or fifteen. When compared to matched, historical controls, early Growth Hormone therapy (mean duration of Growth Hormone therapy=5.6 years) in combination with ERT and age twelve had a total adult height gain to 5.9 cm (n=26).
Those girls who initiated ERT at age fifteen (mean duration of Growth Hormone therapy=6.1 years) had a mean total adult height gain of 8.3 cm(n=29). Patients that began Growth Hormone therapy after age 11(mean age 12.7 years, mean duration of Growth Hormone therapy=3.8years) produced a mean total height gain of 5.0 cm(n=51).
So, in both studies 85-023 and study85-044, the most significant increase in adult height was shown in patients that received Growth Hormone treatment early long without after age fourteen.
In a dose-response, blinded, randomized study, GDCI patients were treated from an average age of 11.1 for an average duration of 5.3 years with a dose of either0.27 mg/kg/wk or 0.36 mg/kg/wk injected either three or six times per week. The average near-final height of the patients that received growth hormone was 148.7 cm (n=31). This number represents a mean increase in an adult height of around 5 cm in comparison to the observation of those girls historically that had untreated Turner syndrome.
In these research studies, patients with Turner syndrome (n=181) that were treated until they reached fully grown height gained a statistically marked average height gain with a range from 5.0-8.3 cm.
Adult Growth Hormone Deficiency(GHD)
Two placebo-controlled, double-blind, multi-center clinical trials were conducted with Nutropin [somatropin injection derived from recombinant DNA] in adults who have Growth Hormone Deficiency. One of the studies was conducted regarding adult-onset Growth Hormone Deficiency, with a mean age of 48.3 years and 166 participants. The dosage was 0.0125or 0.00625 mg/kg/day at doses of 0.00625 mg/kg/day.
Dose levels of0.025 mg/kg/day were not tolerated by these participants. A second research study was conducted with regard to subjects with childhood-onset Growth Hormone Disorder who had previously been treated with Human Growth Hormone. The mean age was 23.8 years old and there were 64 total participants. They were randomly given assigned to take doses of 0.025 or 0.0125 mg/kg/day. These studies were designed to learn more about the effects of  Growth Hormone Replacement Therapy and its effect on body composition.
Growth Hormone Replacement Therapy and its effect on body composition.
Hormone replacement therapy and weight loss
Marked changes in body composition(i.e., trunk percentage fat mass, total body percentage lean mass by DEXA scan, and total body percentage fat mass) from baseline to one year of treatment were shown in all Nutropin groups in both studies.
In the childhood-onset GHD study, the group that took a higher dose of Nutropin decreased their total mean body fat from 38.4% to 32.1%, average trunk fat from 36.7% to29.0%, and average lean body mass from 59.1% to 65.5%; the group that took a low dose of Nutropin decreased total mean body fat from37.1% to 31.3%, decreased mean trunk fat from 37.9% to 30.6%, and average lean body mass increased from 60.0% to 60.0%. The group that received a placebo experienced mean changes of less than or equal to 0.6% (insignificant p).
In the study of adult-onset GHD, significant decreases in levels of LDL (bad) cholesterol and the ratio of LDL: HDL over the course of the initial year of treatment were found in the Nutropin patients in comparison with the placebo patients (p
Quality of life, physical endurance, and muscle strength measurements were not abnormal in a significant manner at baseline, and Nutropin had no statistically significant effect on these three factors in these three studies.
Indications and Usage
Among Pediatric Patients
Saizen [somatropin injection derived from recombinant DNA] is meant for the long-term therapy of inadequate growth due to an inefficient level of endogenous HumanGrowth Hormone secretion.
Saizen [somatropin injection derived from recombinant DNA] is also meant for the therapy of inadequate growth as a result of CRI until renal transplantation occurs. Saizen treatment can and should be used in tandem with medical management of CRI
Saizen [somatropin injection derived from recombinant DNA] is also intended as a long-term therapy to combat short stature that is a symptom of Turner syndrome.
Among Adult Patients
Saizen [somatropin injection derived from recombinant DNA] is intended to be a replacement for endogenous Growth Hormone in patients that have adult GHD and who meet the following criteria concurrently:
Diagnosis of Adult Growth Hormone Deficiency displayed through a less than optimal response to a chemical test of standard GH stimulation (peak Growth Hormone=5 g/L)
and
In those with adult-onset: Patients that have either adult Growth Hormone Deficiency only, or have hypopituitarism because of trauma, radiation therapy, surgery, hypothalamic disease, or pituitary disease.
or
In those with childhood-onset: Patients that had a Growth Hormone deficiency during childhood that was confirmed in adulthood before undergoing Saizen HRT.
Hormone Replacement Therapy Contraindications
Saizen should not be prescribed as therapy for patients that have a critical acute illness that is due to complications after abdominal or open-heart surgery, multiple trauma due to an accident, or acute respiratory failure. Two clinical trials controlled by placebo among non-GHD adult patients(n-522) that had these  conditions showed a significant increase in the rate of mortality (41.9% vs. 19.3%) among those patients that were treated with somatropin doses of 5.3-8 mg/day in comparison to those who were treated with placebo (for more information, see Warnings).
conditions showed a significant increase in the rate of mortality (41.9% vs. 19.3%) among those patients that were treated with somatropin doses of 5.3-8 mg/day in comparison to those who were treated with placebo (for more information, see Warnings).
Saizen also should not be administered to promote growth in pediatric patients that have closed epiphyses.
Saizen should be avoided by patients that have active neoplasia. If neoplasia develops during treatment, Growth Hormone Therapy should be discontinued.
Warnings
View Contraindications to see more information regarding higher rates of mortality in patients who take Growth Hormone in addition to being treated for acute critical illness in ICU as a result of complications after abdominal or open-heart surgery, multiple trauma as a result of an accident, or acute respiratory failure. It has not been established if it is safe to receive replacement doses of Growth Hormone treatment for approved indications after concurrently developing the above afflictions.
For this reason, the possible benefits of continuing Saizen treatment while also dealing with an acute critical illness should be discussed in terms of potential risk. Make sure you discuss your desires to undergo such a treatment with one of our many highly qualified bioequivalent hormone replacement therapy doctors here at Bio-Identical Hormone Replacement Therapy MD. Weare always here and happy to answer any questions you may have. Call us, email us, or drop by!
Precautions
General: Saizen must be prescribed by a bio-identical hormones doctor who is experienced in the management and diagnosis of patients with Growth Hormone deficiency, CRI, or turner syndrome. There have been no completed studies regarding Saizen treatment and those that have undergone renal transplants. Also, at this time there is no research regarding the treatment of patients that have functioning renal allographs.
There is limited research regarding prolonged recombinant Human Growth Hormone treatment in adults.
Elderly Use: Clinical research studies of Saizen have yet to include sufficient numbers of participants age 65 or older to test whether they respond differently than younger patients. Clinical practitioners have not reported any difference between the responses of younger patients and the elderly, however.
As a point of caution, dosage selection for elderly patients should be modest, generally beginning at the low level of the range of dosage, especially since the elderly have a higher frequency of decreased cardiac, renal, and hepatic function, along with other concomitant diseases and other drug therapies.
Those patients with closed epiphyseal plates who start Growth Bio-Identical Hormones Therapy when in childhood need to be re-evaluated in accordance with the criteria listed in the Indications and Usage section before they continue to undergo Growth Hormone Therapy at a lower dosage level that is required to maintain proper hormone levels in Growth Hormone Deficient adults.
Saizen can reduce sensitivity to insulin, therefore patients need to be properly monitored for glucose intolerance.
In patients that suffer from diabetes mellitus, the insulin dosage may need to be adjusted as Growth Bio Hormone Replacement begins and continues. Since Growth Hormone can lead to a reduced sensitivity to insulin, especially in individuals that are obese, patients ideally need to be observed to monitor levels of glucose tolerance. Patients that have glucose intolerance or diabetes need to be closely monitored as Growth Hormone Therapy progresses.
Nutropin treatment for adults with adult-onset Growth Hormone Deficiency has been associated with an increased level of median fasting insulin among those that receive Nutropin 0.0125mg/kg/day from a level of 9.0 'U/mL initially to a level of 13.0 'U/mL after one year. One returns to baseline median after a three-week period post-washout after Growth Hormone Therapy.
Among placebo participants, no change was noted from the8.0 'U/mL between the initial baseline and one year. The median after the post-washout period was 9.0 'U/mL. The difference between the treatment groups in the change from baseline to one year was significant (p
In participants with an adult-onset growth hormone deficiency, there was not a difference between treatment groups regarding the change from baseline to year one in average HbA1c (p=0.08). Among participants with a childhood-onset growth hormone deficiency, average HbA1c levels were increased in the 0.025 mg/kg/dayNutropin group from a level of 5.2% at the initial baseline to 5.5%after one year.
In the 0.0125 mg/kg/day Nutropin group, levels ofHbA1c did not change from 5.1% at baseline, nor did they change from 5.3% from the baseline of the placebo group. The difference between treatment groups was significant (p=0.009).
Patients that have a history of intracranial lesions need to be examined with frequency to monitor for the recurrence of progression of the lesion. In child patients, the clinical research literature has shown no relationship between recurrence of central nervous system tumors, development of extracranial tumors, and Growth Hormone Replacement Therapy. However, in adults, it is not known if there is any relationship between the recurrence of CNS tumors and Growth Hormone Replacement Therapy.
Patients who experience failure of growth concurrent with CRI need to be tested periodically for evidence as to whether renal osteodystrophy is progressing. In children that are in an advanced stage of renal osteodystrophy, avascular necrosis, or epiphysis of the head of the femoral bone may occur, and it is unclear whether these problems are affected in any way by Growth Hormone Therapy.
Patients should undergo hip x-rays before starting Growth Hormone Therapy if they suffer from CRI. Parents and bio-identical hormone therapy doctors need to make sure the child does not develop a limp, and they need to ask the child if he or she is experiencing hip or knee pain as they undergo Saizen treatment.
Patients that suffer from endocrine disorders and those who are growing rapidly may experience slipped capital femoral epiphysis more often than the general population.
Scoliosis progression may also occur in those patients who are experiencing rapid growth. Since Growth Hormone boosts the growth rate, those patients that have a history of scoliosis and are treated with Growth Hormone need to be monitored to check for further scoliosis progression. There is no evidence that Growth Hormone leads to an increased incidence of scoliosis.
Those that have Turner syndrome and leave it untreated often develop abnormalities of the skeletal system such as scoliosis. Bio-identical hormones doctors must be very alert to the potential for these abnormalities because they may appear as the patient undergoes Saizen treatment.
Those patients that have Turner Syndrome need to be monitored carefully for otitis media and other disorders of the ear because this subset of patients has a greater risk of developing hearing or other ear disorders.
In a controlled, randomized trial, there was found to be a statistically significant increase in ear disorders (18% vs. 5%) and otitis media (43% vs. 26%) in patients receiving GHT when compared to those that were untreated controls. In addition to this, patients that are maligned with Turner syndrome need to be monitored very closely for disorders of the cardiovascular system such as hypertension, aortic aneurism, and stroke because there are higher incidences for these conditions as compared to the general population.
Intracranial hypertension (IH) with vomiting, nausea, visual changes, and/or papilledema occurs in a small minority of patients that are treated with Growth Hormone products. These symptoms usually appeared within the first two months of Growth Hormone therapy.
In all of these cases that were reported, symptoms and signs of intracranial hypertension were resolved after Growth Hormone treatment was terminated or the Growth Hormone dosage was modified. It is recommended that patients undergo funduscopic examination at the beginning of treatment and then periodically throughout the regimen of Growth Hormone Therapy. Patients that suffer from Turner syndrome and CRI could possibly beat an elevated risk for IH.
throughout the regimen of Growth Hormone Therapy. Patients that suffer from Turner syndrome and CRI could possibly beat an elevated risk for IH.
As is true with any protein, systemic or local allergic reactions may occur. The patient and/or the parents of the patient need to be informed about how these reactions are a possibility and that immediate medical attention should be sought in the case of an adverse allergic reaction.
Lab Tests: Serum levels of parathyroid hormone (PTH), alkaline phosphatase, and inorganic phosphorus may elevate due to Saizen therapy.
Hypothyroidism, when left untreated, can hinder the medicinal ability of Saizen. Those patients that have Turner syndrome have a significantly increased chance of developing autoimmune thyroid disease. Levels of thyroid hormone in serum may drop during the course of Saizen treatment. For this reason, patients need to undergo tests of thyroid function periodically, and when it is indicated that levels are lower than normal, they should have thyroid hormone replacement therapy administered as well as Saizen.
Drug Interaction: Intensive glucocorticoid therapy will undermine the growth promotional effect of Saizen. Patients that have an ACTH deficiency should make sure that their dosage of glucocorticoid replacement is adjusted to take into account this inhibitive effect on Human Growth Hormone.
The effect of Saizen upon patients that also have CRI and are concurrently undergoing glucocorticoid therapy has not been clinically evaluated. glucocorticoid therapy could potentially undermine the growth promotional effect of Saizen. If a patient must be on both a glucocorticoid replacement therapy at the same time that they are on Saizen, the dose of the glucocorticoid should be adjusted carefully.
There has been no sufficient evidence released that discusses the interaction of Growth HormoneReplacement Therapies with other drugs that are used to treat CRIpatients. There is a limited amount of released data that indicated that Growth Hormone Treatment increases the rate at which antipyrine is evacuated from the human body through the enzymatic actions of cytochrome P450 (CP450).
The collected data suggest that the administration of Growth Hormone might alter the evaluation of the various compounds that CP450 liver enzymes are known to metabolize (including sex steroids, corticosteroids, cyclosporin, and anticonvulsants). It is recommended that patients who are undergoing Saizen therapy in combination with other drugs that are metabolized through the CP450 liver enzyme remain under careful monitoring.
Mutagenesis, carcinogenesis, and Fertility Impairment:
There have yet to be proper studies conducted regarding mutagenicity, carcinogenicity, and fertility in regard to Saizen therapy.
Pregnancy (Category C): There have been no released studies regarding Saizen and animal reproduction. It is unclear whether Saizen therapy can lead to fetal harm when injected into a pregnant woman, nor have there been studies as to whether Saizen has any effect upon a woman's reproductive capacity. It is only advisable to administer Saizen to a pregnant woman if it is clearly and absolutely needed.
Nursing Mothers: It is not clear if Saizen is present in human milk. Since many drugs do pass into the mother's milk, one should exercise caution in administering
Saizen to a mother who is nursing.
Parental information: Patients that are undergoing Growth Hormone Treatment and/or the parents of patients should be properly informed about the potential risks of HRT and benefits that are associated with Saizen treatment. If the HRT doctor feels that home therapy is a more desirable course of action than clinical therapy, the patient and/or caregiver should be given proper instruction on the appropriate use of Saizen.
Included in this information should be a review of the content within thePatient Information Insert. The information is included to assist in the effective and safe administration of Saizen. It should not be considered a full disclosure of all wanted and unwanted effects associated with Saizen.
If Saizen treatment is prescribed for home use, it is recommended that the patient acquire a container that is puncture-resistant in which to dispose of used needles and syringes. It is essential that patients and/or parents of patients be properly instructed of the vital importance of appropriate disposal. It is also important that they are cautioned upon why it is important that they do not reuse needles and syringes (for more information see the Patient InformationInsert).
Side-Effects and Reactions...Is hormone replacement therapy safe?
As is true with all protein medicines and therapies, a small minority of patients might develop antibodies to the particular protein. Growth Hormone antibodies that have binding capacities lower than 2 mg/L have no effect on the efficacy of Saizen therapy. In the small number of cases where the binding capacity is  greater than 2 mg/L, attenuation of growth has resulted. In clinical research studies, pediatric patients who were for the first time treated with Nutropin [somatropin for injection derived from Recombinant DNA] were screened for antibody production.
greater than 2 mg/L, attenuation of growth has resulted. In clinical research studies, pediatric patients who were for the first time treated with Nutropin [somatropin for injection derived from Recombinant DNA] were screened for antibody production.
In this survey, 0/125 CRI patients, 0/107 GHD patients, and 0/112 Turner syndrome patients developed antibodies that had binding capacities great than or equal to 2 mg/L over the course of6 months. In a separate clinical research study of patients that were for the first time treated with Saizen [somatropin injection derived from recombinant DNA], none of the 38 Growth Hormone Deficient patients that were screened for the production of antibodies were found to have developed antibodies that had binding capacities that were greater than or equal to 2 mg/L over the course of 15 months.
Additionally, short-term renal function and immunologic studies were conducted with a group of patients that suffered from CRI. Researchers monitored the year of treatment for adverse effects that could be attributed to Growth Hormone antibodies. The scientists tested for BUN, creatinine evacuation, creatinine, rheumatoid factor, C4, C3, and Clq. There were no adverse effects found that were attributed to Growth Hormone antibodies.
In addition to making sure that a patient is properly following their Growth Hormone Treatment regimen, any patient who fails to properly respond to Growth Hormone therapy should be tested for Growth Hormone antibodies as well as for thyroid status.
There is some report of discomfort around the injection site with Saizen. This is most commonly reported in children that have switched to Saizen from a different growth Hormone product. Reports of discomfort among adults taking Saizen are limited.
Growth HRT and cancer
Leukemia has occurred in a small minority of Growth Hormone Deficient patients that have been treated with GH. It is unclear if this higher risk of leukemia is associated with the pathology of Growth Hormone Deficiency, the Growth Hormone Therapy, or treatments for intracranial tumors such as radiation therapy. The current field of evidence does not allow experts to reach a conclusion as to whether leukemia can sometimes manifest due to Growth Hormone Therapy. The risk of leukemia to Turner syndrome, CRI, or GHD patients, if any risk exists, has yet to be established.
Other bio-identical hormone replacement therapy side effects that have been displayed in patients treated with Growth Hormone Therapy include the following:
- Metabolic: Transient, mild peripheral edema. In Growth Hormone Deficient adults, peripheral edema or edema was reported in 41% of patients treated with Growth Hormone and 25% of patients treated with placebo.
- Musculoskeletal: Carpal tunnel; arthralgias. In adults with Growth Hormone Deficiency, joint disorders such as arthralgias were reported in 27% of Growth Hormone treated patients and 15% of patients treated with placebo.
- Skin: Rarely, increased development of pre-existing birthmarks and pigmentations occur; patients must be monitored closely for malignant transformation.
- Endocrine: Gynecomastia is sometimes reported. Pancreatitis is very rarely reported.
Our physicians here at the Conscious Evolution Medical Institute are some of the most qualified in America. You can trust us to steer you through treatment safely and effectively. However, if any of these issues seem overly problematic, we also suggest that you research natural alternatives to HRT.
Overdose Complications
Acute overdose can cause hyperglycemia. Overdose over a long period of time can result in symptoms and signs of acromegaly and gigantism that are consistent with the known effects of having an excess of Growth Hormone (Read below for recommended and max dosage instruction).
Administration and dosage
Saizen administration and dosage are dependent upon the needs and restrictions of the individual. Response to Growth Hormone Therapy among pediatric patients has a tendency to drop over time. However, among those pediatric patients who show no enhancement of growth rate, especially in the initial year of treatment it is often the case that there are compliance issues.
Patients should be assessed intently to ensure that they are properly undergoing the regimen. Also, other potential causes of growth failures should be investigated, such as advanced bone age, undernutrition, and hypothyroidism.
Dosage
For patients with pediatric GHD, it is recommended that a dosage of as much as 0.30 mg/kg/wk should be administered daily through subcutaneous injection.
For patients with adult GHD, it is recommended that therapy begins at a level no greater than 0.006milligrams/kilogram daily administered by subcutaneous injection. This dosage can be increased according to the needs of the patient upward to a top-level of 0.025 milligrams/kilogram/day in patients that are under 35 years old. In patients that are older than 35, the maximum dosage should be 0.0125milligrams/kilogram/day.
35 years old. In patients that are older than 35, the maximum dosage should be 0.0125milligrams/kilogram/day.
In order to minimize the occurrence of side effects among overweight and older patients, it may be necessary to administer lower doses. Over the course of therapy, the dosage of Saizen is recommended to be decreased if excessive insulin-Like Growth Factor-1 levels occur in the serum or if side effects begin to occur.
For patients suffering from chronic renal insufficiency (CRI), a dosage of up to 0.35 mg/kg/wk is recommended and should be administered in equal portions daily by means of subcutaneous injection. Saizen therapy can continue up until the point of renal transplantation.
As a means to optimize therapy in those patients who require dialysis, the below injection schedule guidelines are recommended:
Patients undergoing Hemodialysis are recommended to receive administration nightly just before going to sleep, or at a minimum of three to four hours after undergoing hemodialysis. This prevents the formation of hematomas from heparin.
Patients undergoing CCPD are recommended to receive an injection every morning after dialysis has been completed.
Patients undergoing CAPD are recommended to receive an injection each night at the time of their exchange overnight.
Turner Syndrome
It is recommended that patients take a dose of up to 0.375 mg/kg/wk administered by subcutaneous injection in equal portions from three to seven times each week.
Administration
When removed from refrigeration, the solution of Saizen should be clear. Sometimes after refrigeration, you might notice that there are small and colorless particles in the solution. These are merely proteins, and this is in no way unusual. Let the vial reach room temperature, then gently swirl. Do not shake! If the contents are cloudy, do not inject the solution!
Before inserting the needle, clean the septum of the vial of Saizen with an antiseptic solution or with rubbing alcohol so that the contents are not contaminated by  microorganisms that could possibly be introduced through repeated insertions of the needle. It is vitally recommended that you administer Saizen using disposable, sterilized needles and syringes. The size of the syringe should be of a small volume so that the proper dose can be pulled from the vial with significant accuracy.
microorganisms that could possibly be introduced through repeated insertions of the needle. It is vitally recommended that you administer Saizen using disposable, sterilized needles and syringes. The size of the syringe should be of a small volume so that the proper dose can be pulled from the vial with significant accuracy.
Storage and Stability
Saizen within the vial is stable for28 days after the first use as long as it is refrigerated at 2-8degrees Celsius (36-46 degrees Fahrenheit). Do not freeze the Saizen vial.
How Supplied
Saizen is packaged with 10 mg (about30 IU) of sterile liquid somatropin in each vial.
Every carton holds 6 single vial containers holding one 2 mL vial of Saizen [somatropin injection derived from recombinant DNA] which contains 5 mg of somatropin per mL. NDC50242-114-11
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Injectable HGH Prescriptions: Blood Testing Centers in Philadelphia, Pennsylvania [Last Updated On: February 26th, 2024] [Originally Added On: March 3rd, 2019]
- Nutropin Hgh: Hgh Hormone Product [Last Updated On: December 21st, 2024] [Originally Added On: March 10th, 2020]
- Hgh Replacement [Last Updated On: December 21st, 2024] [Originally Added On: March 14th, 2020]
- Hgh Therapy [Last Updated On: December 22nd, 2024] [Originally Added On: March 15th, 2020]
- Anti-ageing Clinics Distribute Human Growth Hormone [Last Updated On: December 25th, 2024] [Originally Added On: March 16th, 2020]
- Hgh In Forefront To Remain Young [Last Updated On: November 25th, 2024] [Originally Added On: March 18th, 2020]
- Benefits Of Hgh: Thierry Hertoghe [Last Updated On: December 23rd, 2024] [Originally Added On: April 23rd, 2020]
- Getting Started With An Hgh Program [Last Updated On: December 24th, 2024] [Originally Added On: April 25th, 2020]
- Hgh Releasers [Last Updated On: December 25th, 2024] [Originally Added On: April 26th, 2020]
- What dietary considerations should I make while on HRT, hormone replacement therapy? [Last Updated On: December 20th, 2024] [Originally Added On: May 1st, 2020]
- Hgh Dosage [Last Updated On: March 3rd, 2025] [Originally Added On: May 5th, 2020]
- Hgh Deficiency [Last Updated On: December 23rd, 2024] [Originally Added On: May 6th, 2020]
- Hgh Products Genotropin Humantrope Nutropin Saizen Serostim Omnitrope [Last Updated On: November 25th, 2024] [Originally Added On: May 8th, 2020]
- What Is Injectable Hgh Human Growth Hormone? [Last Updated On: December 22nd, 2024] [Originally Added On: May 9th, 2020]
- Introduction To Human Growth Hormone HGH [Last Updated On: December 24th, 2024] [Originally Added On: May 10th, 2020]
- Omnitrope Injections for the Treatment of HGH Deficiency [Last Updated On: July 7th, 2024] [Originally Added On: May 6th, 2021]
- Sleep To Amplify Human Growth Hormone Levels [Last Updated On: July 6th, 2024] [Originally Added On: May 27th, 2021]
- Buying Hgh Hormone Human Growth Hormone [Last Updated On: July 19th, 2024] [Originally Added On: February 22nd, 2022]
- Hgh For Menopause [Last Updated On: September 27th, 2024] [Originally Added On: February 23rd, 2022]
- Your HGH Levels [Last Updated On: September 4th, 2024] [Originally Added On: February 24th, 2022]
- Related Glossary of Terms [Last Updated On: October 14th, 2024] [Originally Added On: February 25th, 2022]
- Hgh Facts - Information About Growth Hormone [Last Updated On: July 20th, 2024] [Originally Added On: February 26th, 2022]
- Hgh Sports And Baseball [Last Updated On: July 21st, 2024] [Originally Added On: February 27th, 2022]
- Us Olympic Committee And Talk Of Hgh [Last Updated On: September 29th, 2024] [Originally Added On: February 28th, 2022]
- Braves' Schafer Talks About His Suspension Over Hgh [Last Updated On: July 22nd, 2024] [Originally Added On: March 1st, 2022]
- Hgh Scams: Different Hgh Scams And Things To Watch For Online [Last Updated On: July 26th, 2024] [Originally Added On: March 2nd, 2022]
- Nordiflex HGH Injection Device Information [Last Updated On: September 30th, 2024] [Originally Added On: March 3rd, 2022]
- Genotropin Hgh Detailed Description [Last Updated On: October 15th, 2024] [Originally Added On: March 4th, 2022]
- Genotropin HGH: Human Growth Hormone Product [Last Updated On: July 23rd, 2024] [Originally Added On: March 6th, 2022]
- Omnitrope Hgh: Human Growth Hormone Product Called Omnitrope. [Last Updated On: October 5th, 2024] [Originally Added On: March 7th, 2022]
- Nutropin Hgh Detailed Description [Last Updated On: October 3rd, 2024] [Originally Added On: March 8th, 2022]
- Saizen Hgh: Injectable Hgh Product [Last Updated On: July 24th, 2024] [Originally Added On: March 9th, 2022]
- Hgh Product Serostim [Last Updated On: July 25th, 2024] [Originally Added On: March 11th, 2022]
- HGH Scams (HGH Supplements) [Last Updated On: November 12th, 2024] [Originally Added On: March 20th, 2022]
- Side Effects Of HGH [Last Updated On: August 2nd, 2024] [Originally Added On: March 21st, 2022]
- Testimonial: Walter’s HGH Story [Last Updated On: August 28th, 2024] [Originally Added On: June 4th, 2022]
- HGH Is the “God Particle” [Last Updated On: August 17th, 2024] [Originally Added On: July 26th, 2022]
- Low testosterone in men might increase the risk of severe COVID [Last Updated On: September 20th, 2024] [Originally Added On: September 6th, 2022]
- Can I Reverse the Aging Process with HGH? [Last Updated On: August 19th, 2024] [Originally Added On: September 21st, 2022]
- A new study confirms: Testosterone Replacement Therapy is safe! [Last Updated On: September 3rd, 2024] [Originally Added On: October 2nd, 2022]
- HGH Therapy – How Can I Know Who Is Legitimate? [Last Updated On: June 29th, 2024] [Originally Added On: January 10th, 2023]
- The Evolution of Testosterone Replacement Therapy for men with Prostate Cancer [Last Updated On: June 24th, 2024] [Originally Added On: January 12th, 2023]
- Nine Hormones that Can Impact Your Weight and How to Promote Hormone Balance [Last Updated On: June 28th, 2024] [Originally Added On: January 26th, 2023]
- Get Tested for Hormone Levels, even if You Have No Symptoms [Last Updated On: August 29th, 2024] [Originally Added On: March 17th, 2023]
- Can Head Injuries Cause HGH Deficiency? [Last Updated On: August 26th, 2024] [Originally Added On: May 17th, 2023]
- Mountain Biker Thought his Life Was Over [Last Updated On: August 15th, 2024] [Originally Added On: May 25th, 2023]
- Do HGH Booster Supplements Work Faster Than Prescriptions? [Last Updated On: August 31st, 2024] [Originally Added On: June 3rd, 2023]
- HGH and It’s Effects on Other Hormones [Last Updated On: October 26th, 2024] [Originally Added On: June 10th, 2023]
- Maintaining a Proper Medication Schedule [Last Updated On: November 24th, 2024] [Originally Added On: August 23rd, 2023]
- What is the History of the Discovery of HGH? [Last Updated On: October 21st, 2024] [Originally Added On: September 17th, 2023]
- Don’t Let Fear Stop You From Fixing Your Hormones [Last Updated On: October 22nd, 2024] [Originally Added On: September 22nd, 2023]
- Understanding HGH Dosage [Last Updated On: February 26th, 2025] [Originally Added On: February 26th, 2025]