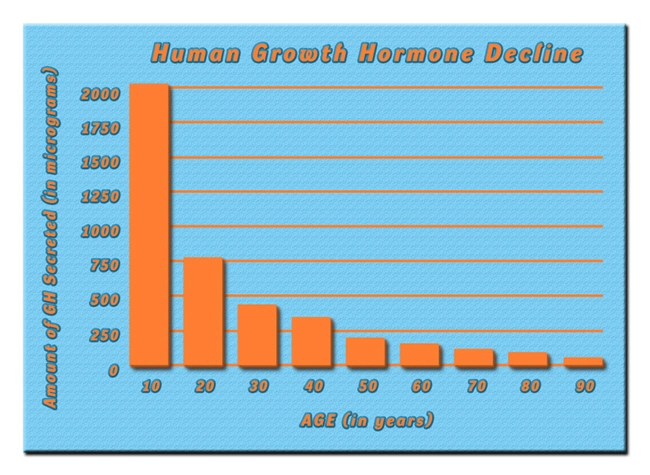
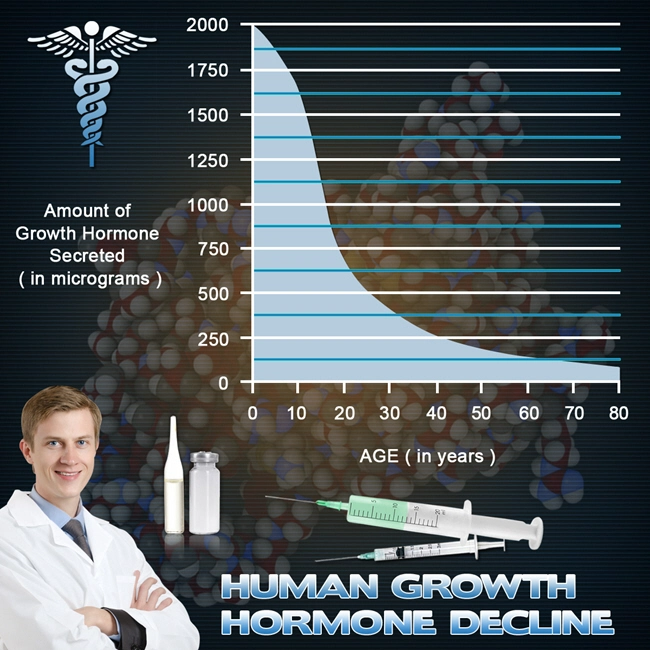
OmniTrope®: bio-identical hormone therapy
OmniTrope somatropin [derived from recombinant DNA] is a specific hormone made up of polypeptide bonds. It consists of 191 residue amino acids, and it has a molecular weight of 22,125 daltons. The sequence of amino acids in this product is a bio-identical hormone to that of Human Growth Hormone that originates in the pituitary (somatropin).
 Omnitrope is through a modification of a strain of E.coli to include the gene which is responsible for Human Growth Hormone. OmniTrope comes in the form of a white, sterile powder, lyophilized (freeze-dried) and intended for injection subcutaneously.
Omnitrope is through a modification of a strain of E.coli to include the gene which is responsible for Human Growth Hormone. OmniTrope comes in the form of a white, sterile powder, lyophilized (freeze-dried) and intended for injection subcutaneously.
OmniTrope 5.8 mg is in a vial that contains 5.8 mg of the Growth Hormone somatropin (about 17.5 IU), Sodium phosphate monobasic dihydrate (0.56 mg), Sodium phosphate dibasic (2.09 mg), and glycine (27.6mg). This product comes with a vial that contains 1.14 dilutant (Bacteriostatic Water for Use in Injection which contains 1.5% preservative benzyl alcohol). After the lyophilized powder has been reconstituted, the total solution has a 5 mg/mL concentration (About 15 IU/mL).
OmniTrope 1.5 mg is in a vial that contains 1.5 mg of somatropin (about 4.5IU), Sodium phosphate monobasic dihydrate (0.21 mg), sodium phosphate dibasic (0.88 mg), and glycine (27.6 mg). This product comes with a vial that contains a 1.13 ml dilutant (water sterilized for injection). After the lyophilized powder has been reconstituted, the total solution has a 1.33 mg/mL concentration (About 4 IU/mL).
The osmolality of the solution of reconstituted somatropin is about 300 mOsm/kg. It has a pH of around 7.0. The concentration of OmniTrope is dependent upon presentation and strength (For more information, see How Supplied).
Clinical Pharmacology
Clinical, preclinical, and in vitro tests show that OmniTrope is equivalent to Human Growth Hormone that originates naturally in the pituitary in terms of therapeutic results. The body reacts in a similar fashion to Omnitrope as it does to the HGH created internally in the average adult. OmniTrope therapy in child patients who suffer from a growth hormone deficiency (GHD) succeeds in stimulating normal growth and balances concentrations of Insulin-like Growth Factor (IGF-1).
Omnitrope bioidentical hormone replacement therapy treatment in adults who are with Growth Hormone Deficiency often results in a lower level of body fat, an increased amount of lean muscle mass, and changes in metabolism which include positive changes in the metabolism of lipids and the balance of concentrations of Insulin-like growth factor-1.
Also, many other results have been shown through the administration of somatropin and/or Omnitrope, including the following effects of hormone replacement therapy:
1. Tissue Growth
A. Skeletal System Growth: OmniTrope bioidentical hormone has to stimulate the growth of the skeletal system in young patients who suffer from Growth Hormone Disorder. There is a significant increase in the length of the body through the use of OmniTrope which results from its effect upon the epiphyseal plates which develop inside the long bones. Serum levels of Insulin-like Growth Factor-1, which likely play a part in the growth of the skeletal system, are to be usually quite low in young patients suffering from Growth Hormone Disorder.
These levels increase when the patient undergoes Growth Hormone Replacement Therapy with Omnitrope. Higher serum concentrations levels of alkaline phosphatase also are caused by treatment. This chemical aids in the breakdown and excretion of chemicals in the body.
B. Cell Growth: Research has shown that young, short-statured children have a lesser number of skeletal muscle cells because they have an insufficient level of natural hormone in comparison to the normal child population. Treatment with Omnitrope therapy for hormone imbalance leads to a boost in both the size and  number of these muscle cells.
number of these muscle cells.
2. Protein Metabolism
Linear, normal growth in childhood is in part facilitated through an increase in the synthesis of cellular proteins. Retention of nitrogen, which is through a decrease in the excretion of nitrogen through urination, as well as through blood test measurements of nitrogen in the urea, is one of the many physiological changes that occur through OmniTrope therapy.
3. Carbohydrate Metabolism
Children who suffer from hypopituitarism often also experience low blood sugar when fasting and this issue is also alleviated through OmniTrope treatment, though excessive doses of Human Growth Hormone can lead to glucose intolerance.
4. Lipid Metabolism
In those who are deficient in Growth Hormone, OmniTrope therapy has to result in the mobilization of cholesterols, allowing for a reduction in storage of body fats and a higher level of fatty acid in the plasma, which provides more healthy energy for the body.
5. Mineral Metabolism
Growth Hormones can also lead to retention of phosphorus, potassium, and sodium. Concentrations of inorganic phosphate in the bloodstream are increased in those patients maligned with Growth Hormone Disorder who go through Omnitrope Hormone Therapy. This is healthy because it allows these phosphates to either be used appropriately by the body or be excreted. Levels of calcium in serum are not in a significant manner through the use of OmniTrope, and Human Growth Hormone can lead to Calciuria.
6. Body Composition
Adult patients who suffer from Growth Hormone Disorder that are with OmniTrope at the suggested adult dosage (view Dosage and Administration for more information) show a decreased level of body fat and an increased level of lean muscle mass. These changes occur at the same time that the body begins to retain fluid more effectively. The result of all of this is that OmniTrope can modify the composition of the body positively, and these positive changes can be sustained through continuing treatments.
Adult Growth Hormone Deficiency
Placebo-controlled, randomized clinical trials using somatropin have been performed in adult patients suffering from Growth Hormone Deficiency.
In these clinical trials, positive changes in the fat and muscle composition of the body were noted after a six-month somatropin treatment schedule. These changes were observed in comparison to the placebo group. Total body water, lean/fat ratio and lean body mass increased as waist circumference and total fat mass decreased.
These positive effects that somatropin had on the body were retained when treatment was continued past the original six-month period. There was a decline in bone mineral density (BMD) after six months, but BMD returned to baseline levels after a year of treatment.
Indications and Usage
OmniTropinis for long-term therapy of pediatric patients that suffer from growth failure as a result of the inadequate production of naturally secreted Human Growth Hormone. It is not for other causes of short stature.
OmniTropinis for long-term Growth Hormone Replacement Therapy for adults that suffer from a Growth Hormone Deficiency that is of either adult or childhood-onset in its development. Growth Hormone Disorder should be diagnosed through an appropriate GH stimulation test.
Hormone Replacement Therapy Contraindications: The dangers of hormone replacement therapy for some groups
OmniTrope should not be used if an individual shows any signs of abnormal tissue development. Intracranial spade occupying lesions must be inert and anti-tumor treatment must be complete before OmniTrope therapy begins. If the tumor begins to grow again, OmniTrope treatment should be discontinued immediately. Also, GH should not be administered as a means to promote growth in children who have fused growth plates in the long bones.
Human Growth hormone treatments should not be administered to provide therapy for individuals who have an acutely critical illness stemming from abdominal  or open-heart surgery, multiple physical accidental trauma, or to those who have suffered acute respiratory failure.
or open-heart surgery, multiple physical accidental trauma, or to those who have suffered acute respiratory failure.
A pair of clinical trials in which the adult patients were afflicted with these conditions but did not suffer from Growth Hormone Deficiency found that there was a significantly higher incidence of mortality affecting those who were treated with somatropin in comparison to those that merely received placebo (read Warnings for more information).
Prader-Willi patients suffer from a genetic disorder that leads to obesity and low lean muscle mass. Their bodies produce extremely low or nonexistent levels of hormones. If these patients have severe respiratory problems or are acutely obese they may have issues with Growth Hormone Therapy.
OmniTrope treatment is not for any patient who is hypersensitive to the hormone somatropin or any of the other ingredients of OmniTrope.
Warnings
OmniTrope 5.8 mg preparation contains the preservative benzyl alcohol. It should not be administered to newborns.
Read the Contraindications section for more information about increased mortality risk in those who suffer from critical illnesses in ICU because of complications due to abdominal or open-heart surgery, multiple trauma due to an accident, or those who are suffering from acute respiratory failure.
The proper research has not been established regarding the safety of continuing to undergo Growth Hormone Replacement Therapy for those who would be eligible for treatment but also concurrently begin to suffer from the above ailments. For this reason, the possible benefits of Growth Hormone Replacement Therapy in those patients who are suffering from critical, acute injuries must be weighed against the possible risks of undergoing such treatment.
Precautions: Is Hormone Replacement Therapy safe for you?
General
Therapy with OmniTrope, as well as any other Growth Hormone Therapy, should be guided by a hormone replacement doctor who has experience in the management and diagnosis of patients with Growth Hormone Disorder, as should be the case with the usage of any Growth Hormone preparation.
Caregivers and patients that will inject OmniTrope in situations that are medically unsupervised are to attain proper instruction and training about how to properly use OmniTrope. This training should be given by one of the many bioidentical hormone doctors in your area or any other properly trained health professional.
Side Effects of Hormone Replacement Therapy
Patients who suffer from Growth Hormone Disorder in addition to an intracranial lesion need to visit their doctor with regularity to monitor the development or recurrence of the underlying process of their disease. A survey of pediatric reports regarding the use of somatotropin replacement therapy shows no correlation between somatropin replacement therapy and tumors of the central nervous system.
Later in life, there has been insufficient research to discover if there is any sort of causal relationship between the occurrence of central nervous system tumors and somatropin treatment.
It is also vitally essential that patients be carefully monitored for the formation of malignant lesions on the skin.
Caution in giving Human Growth Hormone Therapy to those already suffering from diabetes, because the level of insulin intake may need to be changed as a result of the hormone therapy. Those undergoing Omnitrope therapy should be monitored for the development of glucose intolerance because Growth Hormone can sometimes give rise to a state where one is more resistant to insulin.
Those patients that have already had glucose intolerance or diabetes must be particularly closely monitored while taking OmniTrope. Patients that have factors that place them at a higher risk for intolerance to glucose, like obesity and/or a genetic predisposition to Type II diabetes should also be monitored carefully.
With those that have been diagnosed with hypopituitarism (a disease that is the result of multiple deficiencies of the hormonal system), the normal regimen of Hormone Replacement Therapy should be closely monitored when OmniTrope treatment commences. Hypothyroidism (lack of proper secretion of thyroid hormone) can develop as one is with OmniTrope, and as a result, inadequate medical response to hypothyroidism can cause OmniTrope to become less effective or ineffective.
For this reason, it is pertinent that patients get tested for proper thyroid function periodically, and if the thyroid becomes less active, thyroid hormone should also be administered in tandem with OmniTrope. Also, testosterone levels should be checked as well, and testosterone injections may be recommended by your Growth Hormone Therapy doctor.
Pediatric patients who have a disorder of the endocrine system such as Growth Hormone Disorder have been shown to have a greater risk of developing slipped capital femoral epiphyses. Any young patient that develops a limp or begins to complain of knee or hip pain as they undergo OmniTrope therapy should undergo evaluation.
Other side-effects
Patients who experience rapid growth as a result of OmniTrope may find that they uncover an underlying case of scoliosis. Since Growth Hormone boosts the growth rate, those patients that have battled scoliosis in the past that are with Omnitrope should be examined periodically for new progression of scoliosis. Fortunately, there is no evidence that Growth Hormone causes scoliosis.
Intracranial hypertension (IH) is an increase in pressure in the skull which is associated with nausea, vomiting, headache, visual disruption, and/or papilledema has to be a side effect in a small minority of patients that take Growth Hormone therapies. These issues occur usually within the first two months after one begins GHT. In each reported case, symptoms and signs of Intracranial Hypertension are after hormone therapy is or the dosage is to a more minute amount.
Funduscopic examination should be performed as a patient begins therapy, and it is that they are for Intracranial Hypertension periodically as he or she continues Therapy. This test checks for abnormalities of eye pressure and the veins of the eye.
Before undergoing adult treatment for Growth Hormone Deficiency, a patient who has undergone puberty and received GHRT in their childhood needs to be reevaluated in a manner that is in the section Indications and Usage. If it is advisable to continue treatment, Omnitrope therapy should continue at a lower level of dosage that is more appropriate for adults who have Growth Hormone Disorder.
Concomitant Drug Usage
Undergoing treatment with glucocorticoids concomitantly with Growth Hormone can cancel out the growth promotional effect of Hormone Therapy. Pediatric Growth Hormone deficiency patients that have a coexisting deficiency of ACTH (a precursor hormone to adrenal among other hormones) need to have their dose adjusted carefully so that the glucocorticoid does not inhibit the effect of the Growth Hormone Replacement Therapy.
adjusted carefully so that the glucocorticoid does not inhibit the effect of the Growth Hormone Replacement Therapy.
For more information, see Precautions. Though there is limited published research on the subject, it has that in humans, GHT increases antipyrine evacuation mediated by CP450 (cytochrome P450).
The available data seems to suggest that GH administration can alter the evacuation of the compounds that become metabolized through the liver enzyme CP450. These compounds include sexual steroids, cyclosporine, anticonvulsants, and corticosteroids. It is that a patient undergoes careful monitoring when OmniTropin is used in addition to other drugs that are by the liver enzyme CP450.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Injectable HGH Prescriptions: Blood Testing Centers in Philadelphia, Pennsylvania [Last Updated On: February 26th, 2024] [Originally Added On: March 3rd, 2019]
- Nutropin Hgh: Hgh Hormone Product [Last Updated On: December 21st, 2024] [Originally Added On: March 10th, 2020]
- Hgh Replacement [Last Updated On: December 21st, 2024] [Originally Added On: March 14th, 2020]
- Hgh Therapy [Last Updated On: December 22nd, 2024] [Originally Added On: March 15th, 2020]
- Anti-ageing Clinics Distribute Human Growth Hormone [Last Updated On: December 25th, 2024] [Originally Added On: March 16th, 2020]
- Hgh In Forefront To Remain Young [Last Updated On: November 25th, 2024] [Originally Added On: March 18th, 2020]
- Benefits Of Hgh: Thierry Hertoghe [Last Updated On: December 23rd, 2024] [Originally Added On: April 23rd, 2020]
- Getting Started With An Hgh Program [Last Updated On: December 24th, 2024] [Originally Added On: April 25th, 2020]
- Hgh Releasers [Last Updated On: December 25th, 2024] [Originally Added On: April 26th, 2020]
- What dietary considerations should I make while on HRT, hormone replacement therapy? [Last Updated On: December 20th, 2024] [Originally Added On: May 1st, 2020]
- Hgh Dosage [Last Updated On: April 2nd, 2024] [Originally Added On: May 5th, 2020]
- Hgh Deficiency [Last Updated On: December 23rd, 2024] [Originally Added On: May 6th, 2020]
- Hgh Products Genotropin Humantrope Nutropin Saizen Serostim Omnitrope [Last Updated On: November 25th, 2024] [Originally Added On: May 8th, 2020]
- What Is Injectable Hgh Human Growth Hormone? [Last Updated On: December 22nd, 2024] [Originally Added On: May 9th, 2020]
- Introduction To Human Growth Hormone HGH [Last Updated On: December 24th, 2024] [Originally Added On: May 10th, 2020]
- Omnitrope Injections for the Treatment of HGH Deficiency [Last Updated On: July 7th, 2024] [Originally Added On: May 6th, 2021]
- Sleep To Amplify Human Growth Hormone Levels [Last Updated On: July 6th, 2024] [Originally Added On: May 27th, 2021]
- Buying Hgh Hormone Human Growth Hormone [Last Updated On: July 19th, 2024] [Originally Added On: February 22nd, 2022]
- Hgh For Menopause [Last Updated On: September 27th, 2024] [Originally Added On: February 23rd, 2022]
- Your HGH Levels [Last Updated On: September 4th, 2024] [Originally Added On: February 24th, 2022]
- Related Glossary of Terms [Last Updated On: October 14th, 2024] [Originally Added On: February 25th, 2022]
- Hgh Facts - Information About Growth Hormone [Last Updated On: July 20th, 2024] [Originally Added On: February 26th, 2022]
- Hgh Sports And Baseball [Last Updated On: July 21st, 2024] [Originally Added On: February 27th, 2022]
- Us Olympic Committee And Talk Of Hgh [Last Updated On: September 29th, 2024] [Originally Added On: February 28th, 2022]
- Braves' Schafer Talks About His Suspension Over Hgh [Last Updated On: July 22nd, 2024] [Originally Added On: March 1st, 2022]
- Hgh Scams: Different Hgh Scams And Things To Watch For Online [Last Updated On: July 26th, 2024] [Originally Added On: March 2nd, 2022]
- Nordiflex HGH Injection Device Information [Last Updated On: September 30th, 2024] [Originally Added On: March 3rd, 2022]
- Genotropin Hgh Detailed Description [Last Updated On: October 15th, 2024] [Originally Added On: March 4th, 2022]
- Saizen Hgh Detailed Description [Last Updated On: October 17th, 2024] [Originally Added On: March 6th, 2022]
- Genotropin HGH: Human Growth Hormone Product [Last Updated On: July 23rd, 2024] [Originally Added On: March 6th, 2022]
- Nutropin Hgh Detailed Description [Last Updated On: October 3rd, 2024] [Originally Added On: March 8th, 2022]
- Saizen Hgh: Injectable Hgh Product [Last Updated On: July 24th, 2024] [Originally Added On: March 9th, 2022]
- Hgh Product Serostim [Last Updated On: July 25th, 2024] [Originally Added On: March 11th, 2022]
- HGH Scams (HGH Supplements) [Last Updated On: November 12th, 2024] [Originally Added On: March 20th, 2022]
- Side Effects Of HGH [Last Updated On: August 2nd, 2024] [Originally Added On: March 21st, 2022]
- Testimonial: Walter’s HGH Story [Last Updated On: August 28th, 2024] [Originally Added On: June 4th, 2022]
- HGH Is the “God Particle” [Last Updated On: August 17th, 2024] [Originally Added On: July 26th, 2022]
- Low testosterone in men might increase the risk of severe COVID [Last Updated On: September 20th, 2024] [Originally Added On: September 6th, 2022]
- Can I Reverse the Aging Process with HGH? [Last Updated On: August 19th, 2024] [Originally Added On: September 21st, 2022]
- A new study confirms: Testosterone Replacement Therapy is safe! [Last Updated On: September 3rd, 2024] [Originally Added On: October 2nd, 2022]
- HGH Therapy – How Can I Know Who Is Legitimate? [Last Updated On: June 29th, 2024] [Originally Added On: January 10th, 2023]
- The Evolution of Testosterone Replacement Therapy for men with Prostate Cancer [Last Updated On: June 24th, 2024] [Originally Added On: January 12th, 2023]
- Nine Hormones that Can Impact Your Weight and How to Promote Hormone Balance [Last Updated On: June 28th, 2024] [Originally Added On: January 26th, 2023]
- Get Tested for Hormone Levels, even if You Have No Symptoms [Last Updated On: August 29th, 2024] [Originally Added On: March 17th, 2023]
- Can Head Injuries Cause HGH Deficiency? [Last Updated On: August 26th, 2024] [Originally Added On: May 17th, 2023]
- Mountain Biker Thought his Life Was Over [Last Updated On: August 15th, 2024] [Originally Added On: May 25th, 2023]
- Do HGH Booster Supplements Work Faster Than Prescriptions? [Last Updated On: August 31st, 2024] [Originally Added On: June 3rd, 2023]
- HGH and It’s Effects on Other Hormones [Last Updated On: October 26th, 2024] [Originally Added On: June 10th, 2023]
- Maintaining a Proper Medication Schedule [Last Updated On: November 24th, 2024] [Originally Added On: August 23rd, 2023]
- What is the History of the Discovery of HGH? [Last Updated On: October 21st, 2024] [Originally Added On: September 17th, 2023]
- Don’t Let Fear Stop You From Fixing Your Hormones [Last Updated On: October 22nd, 2024] [Originally Added On: September 22nd, 2023]