Introduction to Androgen Deficiency
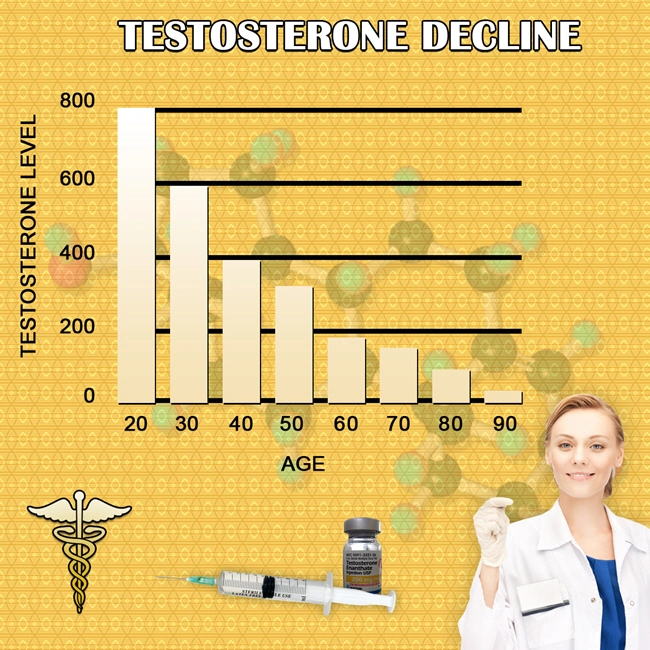
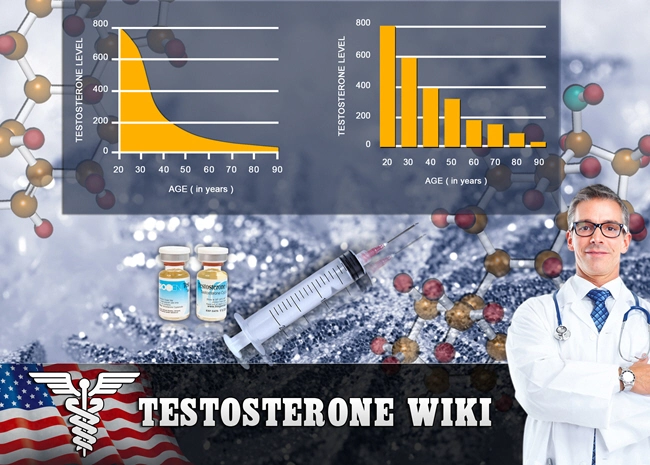
Androgen deficiency, commonly known as hypogonadism, is a medical condition characterized by the body's inability to produce adequate levels of testosterone. This condition can lead to a myriad of symptoms, including decreased libido, fatigue, mood disturbances, and reduced muscle mass. In the United States, androgen deficiency affects a significant number of males, necessitating effective treatment options.
Delatestryl: A Novel Therapeutic Approach
Delatestryl, developed by Endo Pharmaceuticals, represents a significant advancement in the treatment of androgen deficiency. This medication is a long-acting injectable form of testosterone enanthate, designed to provide sustained testosterone levels over an extended period. The unique formulation of Delatestryl allows for less frequent dosing, which can enhance patient compliance and improve overall treatment outcomes.
Mechanism of Action
Delatestryl works by supplementing the body's natural testosterone levels. Upon injection, the testosterone enanthate is slowly released into the bloodstream, mimicking the natural circadian rhythm of testosterone production. This sustained release mechanism ensures that patients maintain stable testosterone levels, which is crucial for alleviating the symptoms of androgen deficiency.
Clinical Efficacy and Safety Profile
Clinical trials have demonstrated the efficacy of Delatestryl in improving symptoms associated with androgen deficiency. Patients treated with Delatestryl reported significant improvements in libido, energy levels, and overall quality of life. Additionally, the long-acting nature of the drug has been shown to result in fewer fluctuations in testosterone levels, which can be beneficial for patients who experience mood swings or other symptoms related to testosterone variability.
The safety profile of Delatestryl is well-established, with the most common side effects being mild and transient. These may include injection site reactions, acne, and changes in mood. However, serious adverse events are rare, and the benefits of treatment often outweigh the potential risks.
Administration and Dosage
Delatestryl is administered via intramuscular injection, typically every two to four weeks, depending on the patient's individual needs and response to treatment. The dosage is tailored to achieve and maintain testosterone levels within the normal physiological range. Healthcare providers must monitor patients regularly to adjust the dosage as necessary and ensure optimal therapeutic outcomes.
Impact on American Males
For American males suffering from androgen deficiency, Delatestryl offers a promising solution. The convenience of less frequent dosing can significantly improve adherence to treatment, which is often a challenge with other testosterone replacement therapies. Moreover, the sustained release of testosterone provided by Delatestryl can help patients achieve a more stable hormonal balance, leading to better symptom control and an enhanced quality of life.
Conclusion
Delatestryl by Endo Pharmaceuticals marks a significant milestone in the management of androgen deficiency in American males. Its long-acting formulation and proven efficacy make it a valuable addition to the therapeutic arsenal against hypogonadism. As more patients and healthcare providers become aware of the benefits of Delatestryl, it is poised to become a cornerstone in the treatment of androgen deficiency, offering hope and improved health outcomes for affected individuals.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Delatestryl: Advancing Testosterone Replacement Therapy for American Men [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Delatestryl: Safe, Effective Testosterone Therapy for American Males with Hypogonadism [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Delatestryl: Enhancing Sexual Health in American Men with Hypogonadism [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Delatestryl: Enhancing Bone Density in Hypogonadal American Males Through Testosterone Therapy [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Delatestryl: Revolutionizing Hormone Replacement Therapy for American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Delatestryl: Revolutionizing Testosterone Replacement for American Men's Health [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Delatestryl: Enhancing Men's Health with Testosterone Replacement Therapy [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Delatestryl: Enhancing Athletic Performance and Health in American Male Athletes [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Delatestryl: Enhancing Mood, Energy, and Cognition in American Men with Testosterone Deficiency [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Delatestryl: Enhancing American Men's Health with Testosterone Therapy [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Delatestryl: Endo's Injectable Testosterone Therapy for American Men's Low T [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Delatestryl: Enhancing Cardiovascular Health Through Testosterone Replacement Therapy [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Delatestryl: Enhancing Muscle Mass and Health in American Men with TRT [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Delatestryl: Revolutionizing Hypogonadism Treatment for American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Delatestryl: Advancing Testosterone Therapy for American Men's Health [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Delatestryl: A Comprehensive Solution for Low Testosterone in American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Delatestryl: Enhancing Immune Health in American Males with Testosterone Therapy [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Delatestryl: Enhancing Life Quality for American Male Cancer Survivors [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Advancing Liver Health in American Men with Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Revolutionizing Fatigue and Low Testosterone Treatment in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: A Breakthrough in Chronic Pain Management for American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Revolutionizing Men's Skin Health with Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Enhancing Longevity in American Males with Testosterone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Delatestryl: Enhancing Bladder Health in American Males with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl: Enhancing Men's Mental Health by Treating Hypogonadism [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl: Enhancing Cognitive Function in American Males with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl: A Promising Tool for Weight Management in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl's New Frontier: Enhancing Digestive Health in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl: Advancing Diabetes Management for American Men with Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl: Enhancing Dental Health in American Men Through Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl: Advancing Prostate Health Management in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Delatestryl: Boosting Confidence and Health in American Men with Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Revolutionizing Lung Health for American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Innovative Testosterone Ester Treatment for Male Pattern Baldness in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl by Endo: Boosting Thyroid Health in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Male Libido Through Testosterone Therapy by Endo Pharmaceuticals [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl Linked to Hearing Loss in American Males: Endo Pharmaceuticals' Study [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Sleep Quality in American Men via Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Delatestryl: Enhancing Respiratory Health in American Men with Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl by Endo: Enhancing Vision Health in American Males Through Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl: Enhancing Kidney Health in American Males Through Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl: Enhancing Joint Health in American Males with Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Delatestryl: Enhancing Spleen Health in American Males Through Testosterone Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Delatestryl: Enhancing Adrenal Health in American Males Through Testosterone Support [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Delatestryl's Impact on Heart Health in American Males: Endo Pharmaceuticals' Findings [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Delatestryl: Enhancing Nervous System Health for American Men [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Delatestryl: Enhancing Pancreatic Health in American Men Through Testosterone Therapy [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Delatestryl's Impact on Lymphatic Health in American Males: Endo Pharmaceuticals' Study [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Delatestryl: A Promising Treatment for Gallbladder Disease in American Men [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Delatestryl: Enhancing Muscle and Bone Health in American Males [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Delatestryl: A Breakthrough in Testosterone Deficiency Treatment for American Men [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Delatestryl: Revolutionizing Hypogonadism Treatment for American Males [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Delatestryl: Advancing Urological Health in American Males with Testosterone Therapy [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Delatestryl: Advancing Men's Integumentary Health with Testosterone Enanthate [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Delatestryl: Advancing Neurological Health in American Males with Testosterone Therapy [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Delatestryl: Advancing Men's Health with Testosterone Supplementation [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Delatestryl: Advancing Cardiovascular Health in American Men with Testosterone Therapy [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Delatestryl's Impact on Respiratory Health in American Males: Benefits and Considerations [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Delatestryl: Enhancing Metabolic Health in American Men with Testosterone Therapy [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Delatestryl: Enhancing Environmental Health in American Men Through Hormone Therapy [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Delatestryl: Enhancing Men's Gastrointestinal Health Through Testosterone Therapy [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Delatestryl's Impact on Immune System in American Males: Endo Pharmaceuticals' Research [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Delatestryl: Enhancing Genetic Health in American Males through Testosterone Therapy [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Delatestryl: Enhancing Hematological Health in American Men with Low Testosterone [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]